
The story of octyl gallate stretches back through more than half a century of food science and materials engineering. Scientists first synthesized this compound in the 1940s as a response to the industrial world’s growing need for stabilizers that could slow down oxidative decay. Postwar food shortages and the push for longer shelf life gave rise to a wave of antioxidants. Octyl gallate, with its origins rooted in gallotannins extracted from oak galls and further modified with octanol, became an answer for food companies and manufacturers looking to beat back the relentless march of rancidity in processed oils and fats. Before synthetic options like octyl gallate stepped onto the scene, producers mostly relied on ascorbic acid, BHT, and tocopherols. Eventually, octyl gallate carved out a niche for itself, especially in products sensitive to more volatile antioxidants.
I remember hearing early accounts from chemists who marveled at how a few grams could change the entire flavor and shelf life of a food batch. Before automation, workers added stabilizers by hand, guided more by experience than calculation. The industrial boom saw octyl gallate’s popularity surge as bottling, canning, and later more complex packaging methods demanded reliable oxidation control. The compound’s commercial production stitched it into the fabric of mass manufacturing, where both cost-saving and quality objectives ruled.
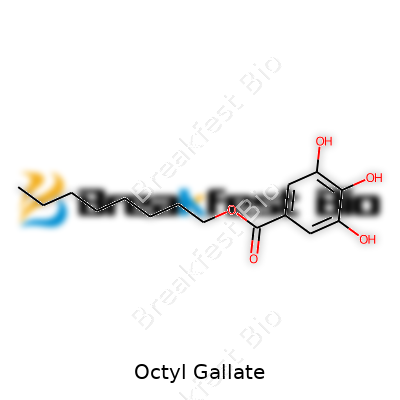
Octyl gallate shows up most often as a white to slightly yellow powder with a faint odor and notably high solubility in alcohol, but it barely dissolves in water. Chemically, it is the ester of gallic acid and 1-octanol, falling under the large umbrella of gallates, each characterized by an ester group. You’ll see it listed under multiple designations, including E311 in Europe’s food additive index, or simply as OG on technical datasheets. Some suppliers label it using synonyms like “1-octyl gallate” or “octyl 3,4,5-trihydroxybenzoate.”
It often travels in woven barrels or lined drums, because it clumps at high humidity, and a tight seal keeps it fresh. Because so many industries rely on accurate reporting, product sheets usually pinpoint purity at 98% or above, with minimal residual solvents. That level of precision speaks to the regulatory scrutiny octyl gallate faces as both a food additive and an industrial stabilizer. You won’t find it in the ingredient list of whole foods, but it sits quietly in the background of processed products where ingredient stability is non-negotiable.
With a molecular formula of C15H22O5 and a molecular weight of about 282.33 g/mol, octyl gallate stands out due to its combination of polar and non-polar groups. The gallic acid backbone brings potent antioxidant power, while the octyl chain adds just enough oil affinity to make it perfect for fats and oils. Its melting point hovers around 100°C, and it starts decomposing instead of truly boiling. That structure means octyl gallate provides a sweet spot between persistence and biodegradability, lingering long enough in products but not sticking around indefinitely.
Technicians appreciate its faint aromatic odor and crystalline nature, which makes it easy to measure out—even in facilities running thousands of batches per month. While its water solubility is nearly negligible, octyl gallate’s solubility shoots up in ethanol and propylene glycol, letting formulators tailor its use to the target system. It resists light and heat better than several other antioxidants, which matters in food processing plants dealing with weather fluctuations and variable storage conditions.
Producers define technical specifications with tight tolerances considering the use in regulated markets. Purity typically exceeds 98%, and heavy metal content stays well below safety thresholds, with lead, mercury, and arsenic down in the parts-per-million or less. The labelling of food-grade octyl gallate draws the line between industrial and edible, stamping clear batch numbers, certification marks, and country-of-origin to trace each shipment down to its batchroom. In the EU, labelling rules require the E311 designation and warnings where used above certain concentrations. US regulators expect even stricter reporting on source, purity, and potential residual solvents.
In everyday manufacturing, clear technical specs cut costs due to fewer batch failures and keep recalls to a minimum. Documentation occupies a surprisingly large share of the day-to-day workflow for both quality assurance and regulatory compliance staff. In regions with stricter import controls, paperwork almost outweighs the product itself.
Octyl gallate starts with the esterification of gallic acid—often derived from plant tannins—and 1-octanol, which can be produced from petroleum or plant oils. Chemists rely on acid catalysis and a controlled environment to push the reaction toward the desired ester. Factors like reaction temperature, catalyst amount, and purification steps influence both yield and final purity. During my time in quality auditing, I toured several facilities where technicians tweaked catalyst concentrations to produce larger grain sizes for industrial applications or finer grains for food-grade batches.
After reaction completion, chemists use liquid-liquid extraction and recrystallization to pull pure octyl gallate from the reaction soup. State-of-the-art facilities automate feeding and purging, while smaller producers may run semi-manual setups, balancing speed and cost. Waste streams containing residual acid, alcohol, and byproducts get neutralized and filtered before discharge. Some labs now use greener solvents and try to minimize water usage—an economic and environmental win.
Octyl gallate, with its three phenolic hydroxyl groups, serves as a strong hydrogen donor, making it a formidable antioxidant. It scavenges free radicals and stabilizes them, preventing propagation of oxidative damage in oils. This property puts it ahead of milder antioxidants in high-fat environments. Storage and processing conditions can kink the octyl chain, causing subtle changes to efficacy; I’ve seen this small shift alter batch results and require recalibration. The compound may also undergo transesterification or hydrolysis, especially at high pH or under strong heating, which forms gallic acid and octanol.
Chemists have explored modifying octyl gallate to create derivatives with altered chain length, hoping to fine-tune solubility and speed of reaction in specialty food systems and cosmetic oils. Research teams work with metal complexes involving octyl gallate, aiming to add antimicrobial power to its antioxidant punch.
Octyl gallate may appear under different trade names and synonyms, depending on supplier and industry. Besides the succinct “octyl gallate,” chemical catalogs use “1-octyl gallate,” “octyl 3,4,5-trihydroxybenzoate,” and “E311.” Some European and American manufacturers package it under branded names, sometimes incorporating hints at its antioxidant function. These variations show up frequently in pharmaceutical ingredient indexes, food additive compendia, and industrial supply lists. Trade language can lead to confusion for new chemists or purchasing agents, who benefit from a broad awareness of the product’s synonyms to avoid doubled orders or regulatory missteps. For years, I built up a spreadsheet of alternative names just to handle supplier diversity and customs paperwork.
Octyl gallate earns a spot on regulatory radar. Agencies classify it as a food additive with clearly defined maximums—usually capped around 100 mg/kg in oils and fats, with lower limits in finished goods. The compound sports a relatively low acute toxicity, but at high doses, studies show mild skin and mucous membrane irritation. Workplace protection standards require gloves, goggles, and proper air handling, mostly because fine dust can irritate eyes or throat. In my experience, plants with open mixing areas usually run local exhaust fans near antioxidant loading ports, minimizing airborne exposure.
Technicians receive specific training on dosing to avoid foaming or over-concentration, which could cause finished product off-flavors or even recalls. Suppliers label each drum or carton with standardized pictograms, precautionary statements, and batch tracking, supporting both workplace safety and downstream traceability. The supply chain has shifted toward digital logs for every step, so if a customer flags an issue, teams can quickly pin down the ingredient’s life story.
Food processing uses octyl gallate to prop up shelf life in oils, fats, margarines, and certain baked goods. Its oil-soluble profile means it works best where water-based antioxidants would fade fast. Chocolate and nut spreads benefit heavily, as the compound guards against the off smells that come with oxidation.
Pharmaceuticals see octyl gallate used to preserve active ingredients in oil-based pills or creams. Cosmetics companies draw on its stability in creams and lotions, leveraging its light resistance to prevent browning in translucent packaging. Industrial applications branch out into lubricants and rubber manufacturing, where it slows down degradation in an environment filled with reactive oxygen species. Some paint and coating manufacturers examine its potential as a stabilizer for specialty formulations.
Academic and industrial scientists take a keen interest in tweaking the molecule for stronger properties or greener production. Recent projects look at incorporating octyl gallate in combination with tocopherol or rosemary extract, hoping for a blend of naturalness and efficacy. Labs evaluate chain-length modifications and look for combinations that would work in both high-moisture and high-fat systems. Nanotechnology opens new routes; researchers try embedding octyl gallate inside polymer films for smart packaging—wrappers that actively shield foods from oxygen penetration.
Pharmaceutical studies investigate the potential use of octyl gallate as a drug excipient, particularly in topical creams designed for antioxidant delivery. In the green chemistry field, teams study more sustainable production, focusing on biocatalysts and low-toxin processing steps.
Toxicological studies from multiple countries, including the US FDA and EFSA in Europe, report a low acute toxicity profile in rodents and other model organisms. Extended exposure studies show that the compound, at regulated food additive levels, does not accumulate in tissues or trigger carcinogenicity. Some murine studies suggest a mild tendency to irritate the gut at high doses, but no clear evidence ties it to chronic disease in standard use ranges. Regulatory monitoring continues as food manufacturers push boundaries in new product launches.
One area where research calls for caution: The interaction with other food additives and processing contaminants sometimes creates more reactive compounds. Scientists call for clearer labelling and real-time data collection to support consumers with allergies or chemical sensitivities.
The journey for octyl gallate isn't over. Global migration toward foods with cleaner labels puts pressure on manufacturers to either prove its safety or replace it with new, naturally-sourced antioxidants. Environmental trends favor green chemistry, so expect more processes using enzymatic esterification or plant-derived raw materials. In packaging, the integration with smart materials holds promise for reducing food waste via antioxidative packaging. Cosmetic and personal care sectors remain hungry for stable, effective antioxidants that can handle temperature swings and light exposure, so octyl gallate’s future looks busy. Companies investing in transparency and cross-industry collaboration stand to lead the evolution of octyl gallate, ensuring continued trust and innovation in this humble but mighty molecule.
A stroll through supermarket aisles shows just how complex ingredient lists have become. Octyl gallate might not jump out like vitamin C or baking soda, but it quietly shapes the shelf life of dozens of familiar products. I noticed it first on the back of a box of cereal. The question hit me: why has this molecule become so common, and what do we know about it?
Oxygen causes foods to go stale. Fats turn rancid, nuts lose crunch, and packaged cereals take on a musty flavor. The food industry has chased after solutions for decades. Octyl gallate works by blocking the free radicals that run wild in fats and oils during storage. It's not the hero anyone writes headlines about, but the difference shows up in fresher-tasting cookies and crackers, even months after baking.
The FDA considers octyl gallate safe in the low concentrations used as an antioxidant, and European authorities have also signed off on it. It appears alongside other antioxidants like BHA and BHT; together, they chip away at food waste and help keep snacks crispy. Some consumers do avoid synthetic additives, which puts a spotlight on transparency and honest labeling. I always tell friends: look up these names instead of getting spooked—most of them stick around because, at certain doses, science keeps coming back with the same answer: risk is low if you use it right.
Octyl gallate steps beyond food products. Skincare creams, lip balms, and personal care products all face oxidation issues—no one wants a moisturizer to smell ‘off’ after a couple months on the vanity. Cosmetic chemists trust octyl gallate as a stabilizer, especially when products contain plant oils and waxes prone to breaking down. Shampoos and conditioners last longer on store shelves, protecting the aromas and ingredients people expect from their daily routines.
It also shows up in some pharmaceuticals, mostly as a preservative. A tablet that resists degradation means no wasted medicine and fewer chances of throwing away expired pills just because an active compound faded away. Call it a small detail, but this kind of value matters, especially in regions where supply lines face delays and medicines sit on warm shelves.
Some folks feel uneasy about synthetic antioxidants, even those regarded as safe. Several food companies now hunt for ‘clean label’ alternatives—think rosemary extract or tocopherols (vitamin E). These tend to cost more and don’t stretch shelf life as much as octyl gallate or its chemical cousins. Supply chain stability leans heavily on affordable, effective stabilizers. Food waste reduction means families save money; food banks distribute more; companies shrink their losses.
Consumer trust comes from brands explaining their ingredient choices, not hiding behind fine print. Allergen checks and transparency around sourcing address the real anxieties: people want to know not just what’s inside, but why it’s there. For me, it comes down to weighing food safety, reducing spoilage, and making sure families see honest labeling regardless of trends. The safest bet: keep listening to current science, weigh risks, and demand info, whether it’s octyl gallate on a snack box or something less familiar in your bathroom cabinet.
Octyl gallate shows up on the label of a surprising range of foods. I remember noticing it first on the back of a loaf of whole wheat bread at the grocery store. Food makers use it as an antioxidant, there to keep oils and fats from going rancid. The taste stays fresh longer, and the shelf life stretches out a bit more.
Curious consumers often want to know, is octyl gallate safe? Years of studies feed into food safety rules. In the United States, the FDA calls octyl gallate "Generally Recognized as Safe" (GRAS) for specific uses. That status carries weight. Scientists and regulators have looked at the available toxicological data. The European Food Safety Authority (EFSA) has also completed a rigourous assessment, reviewing both animal and human evidence. Based on what has come out of those reviews, the typical amounts found in food don’t set off red flags.
In real-world cooking, levels sit far below amounts that cause problems in test settings. Most adults would not reach exposure numbers high enough to matter, even with a varied diet full of packaged foods. That said, research continues. Data from both animal and human studies informs ongoing monitoring, with extra caution for kids or people with special diets.
That being said, caution serves a purpose. Rare cases of allergic reactions exist, such as hives or mild stomach issues after someone eats foods with octyl gallate. Some people watch their intake of food additives in general, either out of concern or because of specific health conditions. Processed food often comes with a mix of preservatives. Some studies in rodents raise questions about high doses—much higher than anyone would get from normal food. Those animal studies remind scientists to keep up surveillance, especially as food habits change worldwide.
I’ve talked with people who try to avoid additives in all forms, sticking with homemade bread or snacks. Others look at the data on food safety and trust their local authorities to keep things in check. Both approaches have value. Having open information gives everyone the chance to make a choice that fits their needs.
People want to feel safe about what’s in their food. That trust in food safety builds on clear evidence and transparency from regulators and companies. Octyl gallate is just one of a long list of additives, and each one comes under scrutiny through periodic reviews and updated research. As our food systems evolve, new information gets folded into public guidelines. Food allergies, changing consumption patterns, and unique dietary needs all play into how safety gets measured and managed.
Seeking out fresh or less-processed foods naturally lowers additive intake. Store brands bring out new lines marked “free from” for those who prefer fewer synthetic ingredients. At the same time, keeping up with live research matters. Public agencies and independent scientists run ongoing studies and communicate new findings whenever they appear. More transparency on labels and easy access to up-to-date research let families make stronger choices.
Taking responsibility for what goes into each meal matters. Some people rely on baked foods and shelf-stable snacks, especially in busy households or communities with limited access to fresh groceries. Finding a practical balance between safety and convenience forms a reality for many. Risk so far appears low at typical levels, but asking good questions and understanding what’s on the label never goes out of style.
Octyl gallate turns up in all sorts of places in our food and cosmetics. You might spot it on an ingredients label for baked goods or find it in lotions. Its job mostly involves slowing down the fats and oils from going rancid too soon. For most people picking up a cake at the store, few are thinking about the preservatives working in the background. But just because something keeps food fresh for longer doesn’t mean it comes without risks.
A handful of side effects have caught the attention of scientists and watchdogs. Some people have reported allergic reactions, especially those sensitive to preservatives. These reactions might look like itchy skin, trouble breathing, or other typical allergy symptoms. Though rare, such responses often remind us that additives can carry hidden risks, especially for those who already deal with allergies or asthma.
Concern isn’t limited to rare reactions. Several studies in animals suggest high doses can cause mild irritation to the stomach. Rats fed with large amounts over time sometimes end up with some upset-looking intestinal tissue. The numbers used in experiments often far exceed what humans encounter through food, but the research signals a need to pay attention to cumulative exposure.
British researchers looked at food preservatives, including octyl gallate, and raised questions about links to hyperactivity in kids. The evidence sits on the thin side, though, according to regulatory agencies in the US and Europe. Still, it’s tough to ignore the stories from parents who notice their child acting out after a snack loaded with processed ingredients. Anecdotes like those push scientists to dig deeper, since what happens in the lab doesn’t always match up with real life.
Regulators keep an eye on additives, including octyl gallate. The US Food and Drug Administration includes it on the list of ingredients considered safe, but only if used within specific limits. European authorities set similar boundaries. Realistically, the average person isn’t likely to break past those levels through an ordinary diet. Most folks would have to go out of their way to eat enough processed food to build up a risky amount in their system. The trouble comes when someone routinely leans on processed foods or already deals with allergies.
People looking to skip out on octyl gallate or other preservatives might focus more on whole foods and simple ingredient lists. Cooking at home with fresh produce and single ingredients can help cut down on chemical exposure. Some parents pay extra attention to snack foods and cereals, especially if their kids deal with allergies or jittery behavior. Allergy panels and discussions with a doctor can help flag sensitivities, and most food manufacturers will provide ingredient data if someone asks directly.
As the science keeps moving forward, so does the conversation about food safety. Staying curious, checking labels, and sharing experiences with your healthcare provider often work better than letting concern over additives slide into the background.
Octyl gallate pops up on ingredient lists more often than some folks notice. Used as an antioxidant, this additive helps prevent fats and oils from turning rancid in foods like margarine, baked goods, and chewing gum. For years, people have looked at ingredient panels and scratched their heads about what each chemical does to their health. I’ve seen enough confusion from friends and family who want to know if something is safe. Honestly, it's a fair concern. So, I took a deeper dive into where octyl gallate lands with the people who set food and safety rules.
American shoppers come across octyl gallate under its code E311. In the United States, the Food and Drug Administration (FDA) gave it a green light for use as a food additive. FDA’s approval lets food makers use specific amounts to keep lipid-rich foods fresh longer. The FDA draws its line from research that assesses both short-term and long-term safety before anything gets added to the food supply.
Outside the US, regulatory bodies in Europe—specifically, the European Food Safety Authority (EFSA)—keep their own close watch. The European Union permits octyl gallate as an antioxidant, also with maximum levels to avoid any chance of overdoing it. They pay attention to new findings and periodically update their guidance based on ongoing research. Australia and New Zealand accept it as well, taking cues from scientific data and peer regulatory offices.
Approving an additive like this involves more than a quick review. Both FDA and EFSA evaluate animal studies, laboratory data, and sometimes human trials. They look into toxicity, possible links to allergic responses, and whether the body breaks the compound down without trouble. I’ve learned in my science reporting years that most rejections happen when additives leave questions about cancer risk, reproductive effects, or cause unexplained symptoms. So far, the evidence for octyl gallate doesn’t set off these alarms, as long as people keep their daily intake below the established threshold.
That being said, research from past decades suggested rare cases of skin sensitivity or mild allergies when octyl gallate was used in cosmetics. Food use, though, involves much smaller exposures than, say, skincare or direct contact. The lack of widespread health complaints in populations where octyl gallate is allowed gives experts more confidence about its safety in food.
Curiosity about what lands in processed foods shows people want to make informed decisions. Health-conscious friends of mine will pull out their phones and look up food codes or chemical names while shopping. The problem comes when technical language in regulatory approval reports leaves regular folks confused. Communication failure breeds more fear. What could help is clearer labeling, open data from safety studies, and better translation of food science for the public.
Food technology brings real benefits, especially by cutting down food waste or extending shelf life. People deserve transparency about what’s being added and what science says about those choices. Strong approval by agencies like the FDA and EFSA tells me that octyl gallate, within prescribed limits, stands as a safe option. At the same time, authorities and industry don’t get a free pass. They need to keep revisiting old decisions with fresh data, making room for changes as new science comes in. Public trust depends on it, more than any technical certification ever could.
Octyl gallate stands out in the world of antioxidants. If you have ever glanced at the ingredient list of snacks, cooking oils, or even cosmetic products, you might have seen it. Chemically, octyl gallate forms when gallic acid reacts with 1-octanol through a process known as esterification. It doesn’t take a chemist’s degree to notice that octyl gallate has two notable groups: the gallate part, which comes from gallic acid, and an octyl chain made of eight carbon atoms, all linked together and ending with a methyl group. The chemical formula is C15H22O5. Its structure features a benzene ring with three hydroxyl groups sitting at positions 3, 4, and 5. Attached to that ring through a carboxyl group, there's an octyl chain, which gives it a unique combination of water-loving and oil-loving properties.
There’s no mistaking that the structure of octyl gallate does a lot more than look pretty in a textbook. Those three hydroxyl groups attached to the benzene ring give it strong antioxidant properties. They give away hydrogen atoms easily, neutralizing free radicals in fats or oils, and that helps slow down the spoilage of food. The long octyl chain, on the other hand, gives the molecule its ability to glide smoothly into oily environments — not something you get with gallic acid alone. I’ve seen this dual-sided nature pay off in bakeries, where octyl gallate gets picked for extending shelf life without affecting taste or consistency much. The science supports this too; studies show it’s more effective in fats and oils compared to other antioxidants that lack this long hydrocarbon tail.
Take personal care products, like lotions or shampoos. Oils and water need to behave for textures to stay smooth, and the structure of octyl gallate helps stabilize blends of oil and water. It does this through its amphipathic personality: one end loving water, the other cozying up with oils. That makes the product less likely to turn rancid on the shelf. Food manufacturers also lean on this feature, since it keeps flavor and quality intact in nuts, chips, and processed foods.
Comparing octyl gallate to butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT) shows real differences. Both BHA and BHT lack the same number of hydroxyl groups, which limits their antioxidant punch in certain fats, especially under heat. Octyl gallate’s structure offers better performance in warm frying oils, where cheap antioxidants can fall short. This means fewer off-flavors and healthier products for folks who snack regularly or cook with oils at high temperatures.
No chemical comes without debate or scrutiny. Studies continue to test the safety limits of food additives like octyl gallate. Regulatory agencies such as the FDA in the United States and EFSA in Europe have set acceptable daily intake levels, keeping food safety in check. They base decisions on both the molecule’s behavior inside bodies and its track record on supermarket shelves. Responsible sourcing matters too. Companies need transparency—knowing where each batch comes from and how much makes it into finished products adds trust at every step.
Octyl gallate’s unique structure brings together science and everyday living. It proves that the architecture of a molecule can ripple through food safety, product quality, and shelf stability in ways that affect both businesses and families. Choosing effective antioxidants based on structure helps deliver cleaner labels and safer foods, especially as more shoppers want to know what’s in their kitchen and why it works.
| Names | |
| Preferred IUPAC name | octyl 3,4,5-trihydroxybenzoate |
| Other names |
n-Octyl gallate
Octyl 3,4,5-trihydroxybenzoate 1-Octyl gallate Gallic acid octyl ester Octylgallat |
| Pronunciation | /ˈɒk.tɪl ˈɡæl.eɪt/ |
| Identifiers | |
| CAS Number | 103-26-4 |
| Beilstein Reference | 859754 |
| ChEBI | CHEBI:7755 |
| ChEMBL | CHEMBL1406 |
| ChemSpider | 5947 |
| DrugBank | DB06716 |
| ECHA InfoCard | EC Number 204-886-1 |
| EC Number | EC 204-886-1 |
| Gmelin Reference | 70224 |
| KEGG | C06816 |
| MeSH | D017360 |
| PubChem CID | 12385 |
| RTECS number | MD9600000 |
| UNII | 805HR35W1U |
| UN number | UN3082 |
| Properties | |
| Chemical formula | C15H22O5 |
| Molar mass | 460.61 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.2 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 2.8 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 10.69 |
| Basicity (pKb) | 8.97 |
| Magnetic susceptibility (χ) | -7.68E-6 cm^3/mol |
| Refractive index (nD) | 1.482 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 577.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1318.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7805.4 kJ/mol |
| Pharmacology | |
| ATC code | A01AB11 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H317 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0- Ansprechpartner |
| Flash point | Flash point: 210 °C |
| Autoignition temperature | 410 °C |
| Lethal dose or concentration | LD50 (oral, rat): 3800 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 5,000 mg/kg |
| NIOSH | WJ2000000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 150 mg/kg |
| Related compounds | |
| Related compounds |
Propyl gallate
Ethyl gallate Butyl gallate Gallic acid |